Materials can be classified depending on whether they allow charge to move. If charge can easily move through a material, such as metals, then these materials are called conductors. This means that charge can be conducted (i.e., move) through the material rather easily. If charge cannot move through a material, such as rubber, then this material is called an insulator.
Most materials are insulators. Their atoms and molecules hold on more tightly to their electrons, so it is difficult for electrons to move between atoms. However, it is not impossible. With enough energy, it is possible to force electrons to move through an insulator. However, the insulator is often physically destroyed in the process. In metals, the outer electrons are loosely bound to their atoms, so not much energy is required to make electrons move through metal. Such metals as copper, silver, and aluminum are good conductors. Insulating materials include plastics, glass, ceramics, and wood.
The conductivity of some materials is intermediate between conductors and insulators. These are called semiconductors. They can be made conductive under the right conditions, which can involve temperature, the purity of the material, and the force applied to push electrons through them. Because we can control whether semiconductors are conductors or insulators, these materials are used extensively in computer chips. The most commonly used semiconductor is silicon. The chart below shows various materials arranged according to their ability to conduct electrons.
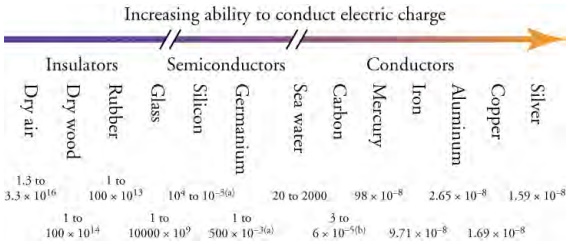
Materials can be arranged according to their ability to conduct electric charge. The slashes on the arrow mean that there is a
very large gap in conducting ability between conductors, semiconductors, and insulators, but the drawing is compressed to fit on
the page. The numbers below the materials give their resistivity in Ω•m (which you will learn about below). The resistivity is a
measure of how hard it is to make charge move through a given material.
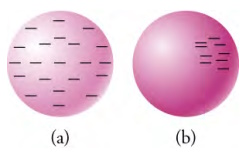
(a) A conducting sphere with excess negative charge (i.e., electrons). The electrons repel each other and spread out to cover
the outer surface of the sphere. (b) An insulating sphere with excess negative charge. The electrons cannot move, so they remain
in their original positions.
What happens if an excess negative charge is placed on a conducting object? Because like charges repel each other, they will push against each other until they are as far apart as they can get. Because the charge can move in a conductor, it moves to the outer surfaces of the object. The the left side of the picture above shows schematically how an excess negative charge spreads itself evenly over the outer surface of a metal sphere.
What happens if the same is done with an insulating object? The electrons still repel each other, but they are not able to move, because the material is an insulator. Thus, the excess charge stays put and does not distribute itself over the object. The the right side of the picture above shows this situation.
Transfer and Separation of Charge
Most objects we deal with are electrically neutral, which means that they have the same amount of positive and negative charge. However, transferring negative charge from one object to another is fairly easy to do. When negative charge is transferred from one object to another, an excess of positive charge is left behind. How do we know that the negative charge is the mobile charge? The positive charge is carried by the proton, which is stuck firmly in the nucleus of atoms, and the atoms are stuck in place in solid materials. Electrons, which carry the negative charge, are much easier to remove from their atoms or molecules and can therefore be transferred more easily.
Electric charge can be transferred in several manners. One of the simplest ways to transfer charge is charging by contact, in which the surfaces of two objects made of different materials are placed in close contact. If one of the materials holds electrons more tightly than the other, then it takes some electrons with it when the materials are separated. Rubbing two surfaces together increases the transfer of electrons, because it creates a closer contact between the materials. It also serves to present fresh material with a full supply of electrons to the other material. Thus, when you walk across a carpet on a dry day, your shoes rub against the carpet, and some electrons are removed from the carpet by your shoes. The result is that you have an excess of negative charge on your shoes. When you then touch a doorknob, some of your excess of electrons transfer to the neutral doorknob, creating a small spark.
In the top row, a metal sphere with 100 excess electrons transfers 25 electrons to a metal sphere with an excess of 50 electrons. After the transfer, both spheres have 75 excess electrons. In the bottom row, a metal sphere with 100 excess protons receives 25 electrons from a ball with 50 excess protons. After the transfer, both spheres have 75 excess protons.
Touching the doorknob with your hand demonstrates a second way to transfer electric charge, which is charging by conduction. This transfer happens because like charges repel, and so the excess electrons that you picked up from the carpet want to be as far away from each other as possible. Some of them move to the doorknob, where they will distribute themselves over the outer surface of the metal. Another example of charging by conduction is shown in the top row of the chart above. A metal sphere with 100 excess electrons touches a metal sphere with 50 excess electrons, so 25 electrons from the first sphere transfer to the second sphere. Each sphere finishes with 75 excess electrons.
The same reasoning applies to the transfer of positive charge. However, because positive charge essentially cannot move in solids, it is transferred by moving negative charge in the opposite direction. For example, consider the bottom row of Figure 18.10. The first metal sphere has 100 excess protons and touches a metal sphere with 50 excess protons, so the second sphere transfers 25 electrons to the first sphere. These 25 extra electrons will electrically cancel 25 protons so that the first metal sphere is left with 75 excess protons. This is shown in the bottom row of the chart above. The second metal sphere lost 25 electrons so it has 25 more excess protons, for a total of 75 excess protons. The end result is the same if we consider that the first ball transferred a net positive charge equal to that of 25 protons to the first ball.
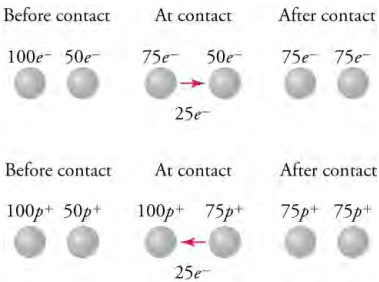
In the top row, a metal sphere with 100 excess electrons transfers 25 electrons to a metal sphere with an excess of 50
electrons. After the transfer, both spheres have 75 excess electrons. In the bottom row, a metal sphere with 100 excess protons receives
25 electrons from a ball with 50 excess protons. After the transfer, both spheres have 75 excess protons.
In this discussion, you may wonder how the excess electrons originally got from your shoes to your hand to create the spark when you touched the doorknob. The answer is that no electrons actually traveled from your shoes to your hands. Instead, because like charges repel each other, the excess electrons on your shoe simply pushed away some of the electrons in your feet. The electrons thus dislodged from your feet moved up into your leg and in turn pushed away some electrons in your leg. This process continued through your whole body until a distribution of excess electrons covered the extremities of your body. Thus your head, your hands, the tip of your nose, and so forth all received their doses of excess electrons that had been pushed out of their normal positions. All this was the result of electrons being pushed out of your feet by the excess electrons on your shoes.
This type of charge separation is called polarization. As soon as the excess electrons leave your shoes (by rubbing off onto the floor or being carried away in humid air), the distribution of electrons in your body returns to normal. Every part of your body is again electrically neutral (i.e., zero excess charge).
The phenomenon of polarization is seen in. The child has accumulated excess positive charge by sliding on the slide. This excess charge repels itself and so becomes distributed over the extremities of the child’s body, notably in his hair. As a result, the hair stands on end, because the excess negative charge on each strand repels the excess positive charge on neighboring strands.
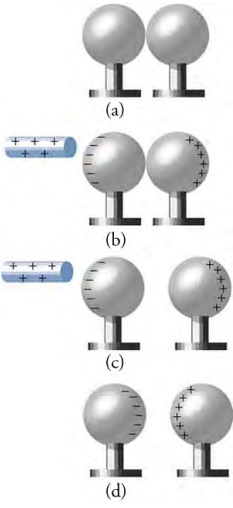
(a) Two neutral conducting spheres are touching each other, so the charge is evenly spread over both spheres. (b) A positively
charged rod approaches, which attracts negative charges, leaving excess positive charge on the right sphere. (c) The spheres are
separated. Each sphere now carries an equal magnitude of excess charge. (d) When the positively charged rod is removed, the excess
negative charge on the left sphere is attracted to the excess positive charge on the right sphere.
Polarization can be used to charge objects. Consider the two metallic spheres shown in the image above part a. The spheres are electrically neutral, so they carry the same amounts of positive and negative charge. In the top picture the image above, the two spheres are touching, and the positive and negative charge is evenly distributed over the two spheres. We then approach a glass rod that carries an excess positive charge, which can be done by rubbing the glass rod with silk, as shown in the image above part b. Because opposite charges attract each other, the negative charge is attracted to the glass rod, leaving an excess positive charge on the opposite side of the right sphere. This is an example of charging by induction, whereby a charge is created by approaching a charged object with a second object to create an unbalanced charge in the second object. If we then separate the two spheres, as shown in the image above part c, the excess charge is stuck on each sphere. The left sphere now has an excess negative charge, and the right sphere has an excess positive charge. Finally, in the bottom picture, the rod is removed, and the opposite charges attract each other, so they move as close together as they can get.
Article source: OpenStax is a nonprofit educational technology initiative based at Rice University. OpenStax's mission is to improve educational access and learning for everyone. Textbooks on OpenStax's site are licensed under a Creative Commons Attribution 4.0 International License.

