Atoms are the building blocks of all matter. Different kinds of atoms are referred to as "elements", and they are compiled into a chart called the periodic table. The table allows similar elements to be grouped together based on their chemical properties. Interestingly, atoms in the same group often have similar physical properties as well. If you want to gain an understanding of the chemical and physical properties of atoms, learn to divide the periodic table into groups and study each the properties of each group.
Dividing the Periodic Table
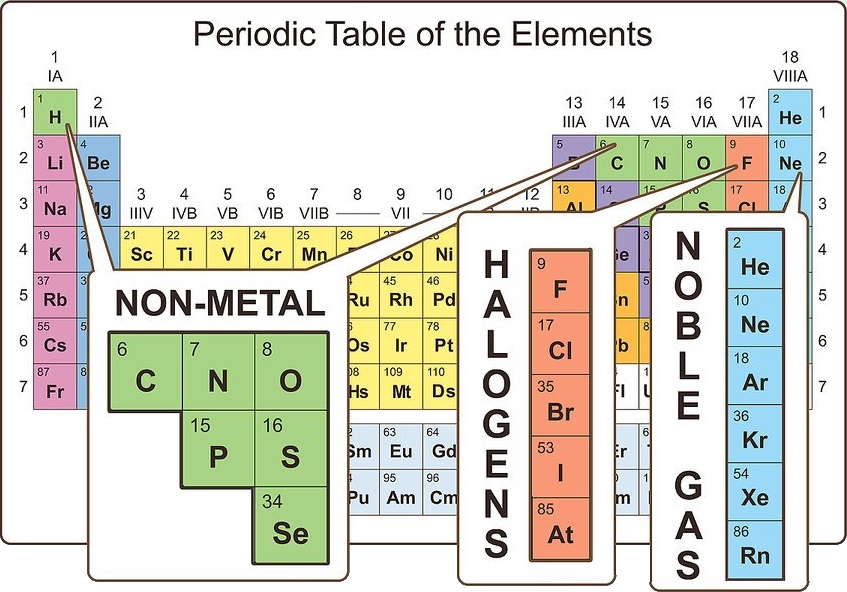
Locate the elements that are nonmetals
On the periodic table, the majority of atoms are classified as metals. Other atoms are classified as nonmetals. You will find these groupings beneficial as you explore the properties of different atoms.
Nonmetals can be found primarily in the upper right corner of the periodic table, while the rest of the table consists primarily of metals. Hydrogen is an exception to this rule, as it acts like a nonmetal under standard conditions, but it is found in the upper left corner of the table.
Carbon, nitrogen, oxygen, hydrogen, sulfur, and noble gases (the elements in the far right column) are commonly known nonmetals. Halogens (such as fluorine, chlorine, bromine, etc.) fall into the nonmetal category.
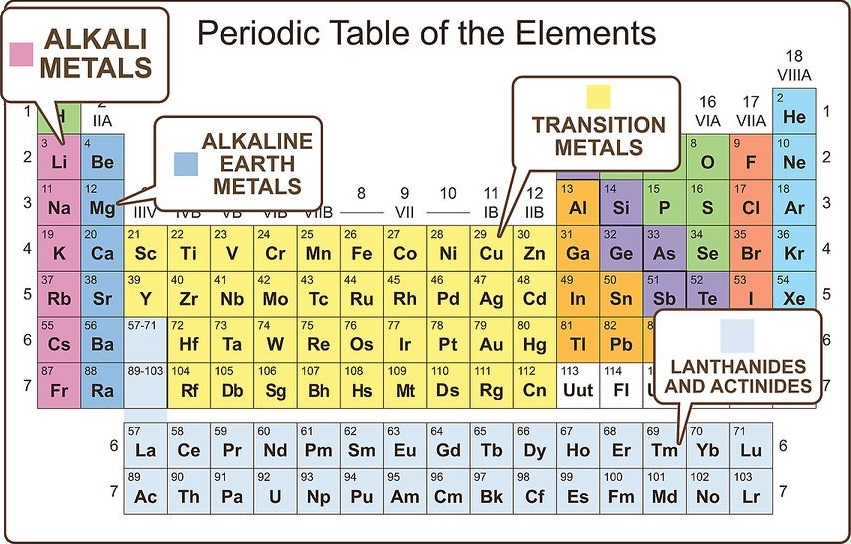
Differentiate the major metal groupings
Metals are grouped into subcategories. Elements within these subcategories are alike in more specific ways than just identifying both as metals. The common categories are alkali metals, alkaline earth metals, transition metals, post-transition metals, lanthanides, and actinides.
Alkali metals are very reactive and readily ionize to a 1+ state.
Alkaline earth metals are slightly less reactive, but readily ionize to a 2+ state.
Transition and post transition metals are more stable and have many different ionization states.
Lanthanides and actinides are larger, less stable molecules that readily react. Some of them decompose, making them radioactive.
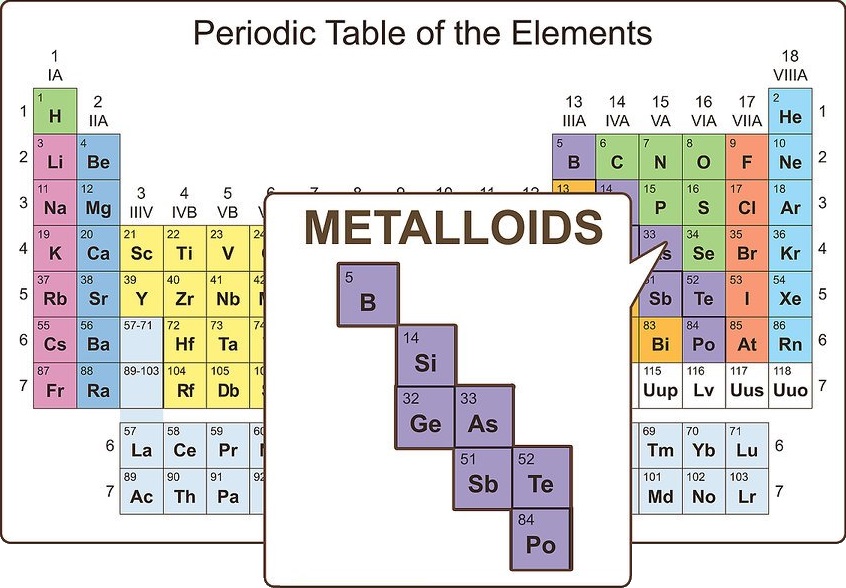
Imagine something between a metal and nonmetal
Such elements do exist, and they are known as metalloids. On the periodic table, metalloids show up between post-transition metals and nonmetals. There are eight metalloids:
• Boron
• Silicon
• Germanium
• Arsenic
• Antimony
• Tellurium
• Polonium
• Astatine
Analyzing Chemical Characteristics
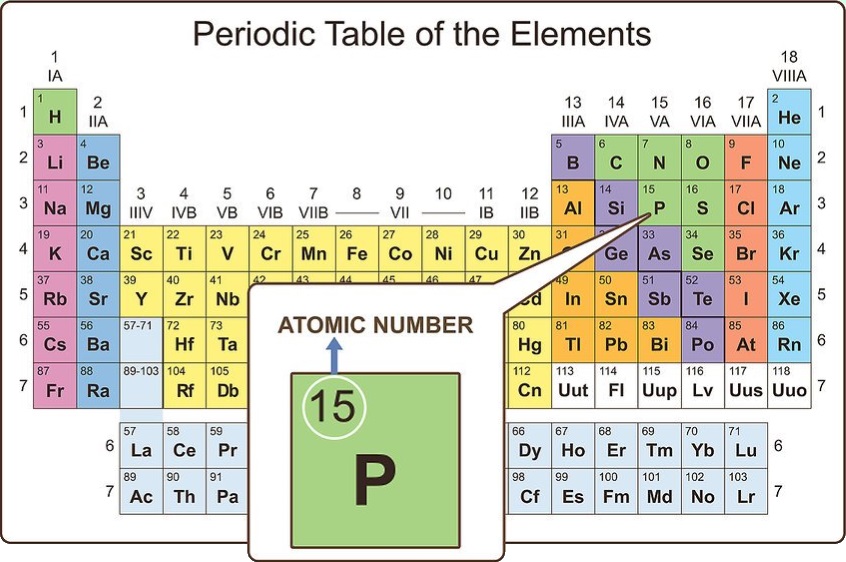
Look at the order of the table
When you look at the periodic table, you will notice that the elements are all numbered. This numbering is far from random. Actually, it is known as the atomic number for that particular element and is equal to the number of protons the element has in its nucleus.
For atoms (not ions), the atomic number also represents the number of electrons in the atom. The number of protons and electrons in an atom are equal. On some periodic tables, you will notice a second number that is in a smaller font. This is the average atomic mass for that element.
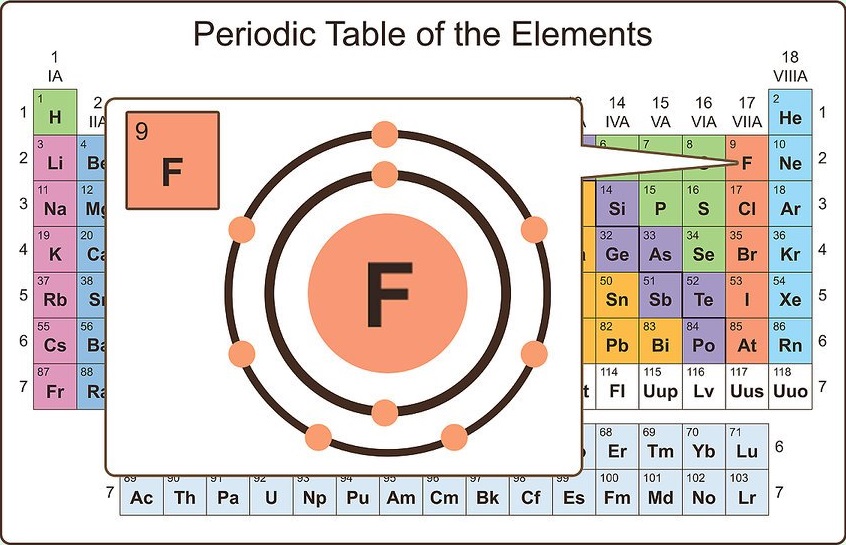
Visualize valence electrons
Valence electrons are the electrons in the outermost shell of an atom's electron cloud. The valence electrons are the single largest factor in how the atom will react chemically. The most stable configuration for an atom is to have the electrons in its outer shell filled, therefore it will not bond with other atoms.
In most cases, the outer shell needs to contain eight electrons to be full (depending on the size of the atom this can vary). For example, fluorine has nine electrons. The first two fill the innermost orbital, the remaining seven are valence electrons. This means fluorine needs only one more electron to fill its valence shell. Thus, fluorine readily reacts with atoms that can give up an electron (particularly metals).
An example of the opposite is lithium. Lithium has three electrons. The first two fill the innermost shell and the last one is a valence electron. Since lithium would need to gain seven electrons to fill its valence shell, it is easier (more energetically favorable) for it to shed the one valence electron it has instead. So, lithium readily reacts with elements that will accept an electron (like halogens).
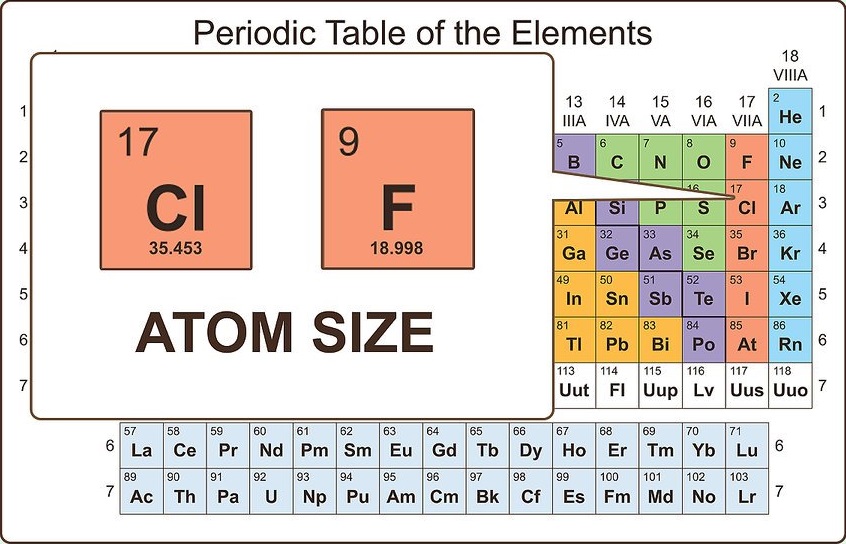
Consider the size of the atom
Though the valence electrons are the best predictor of chemical properties in a given atom, the size of the atom also matters. Larger atoms have more electrons between the nucleus and valence electrons, which means that they are held to the atom more loosely than on smaller atoms. This accounts for why two atoms with the same number of valence electrons (for example, fluorine and chlorine) have similar, but not identical, chemical properties.
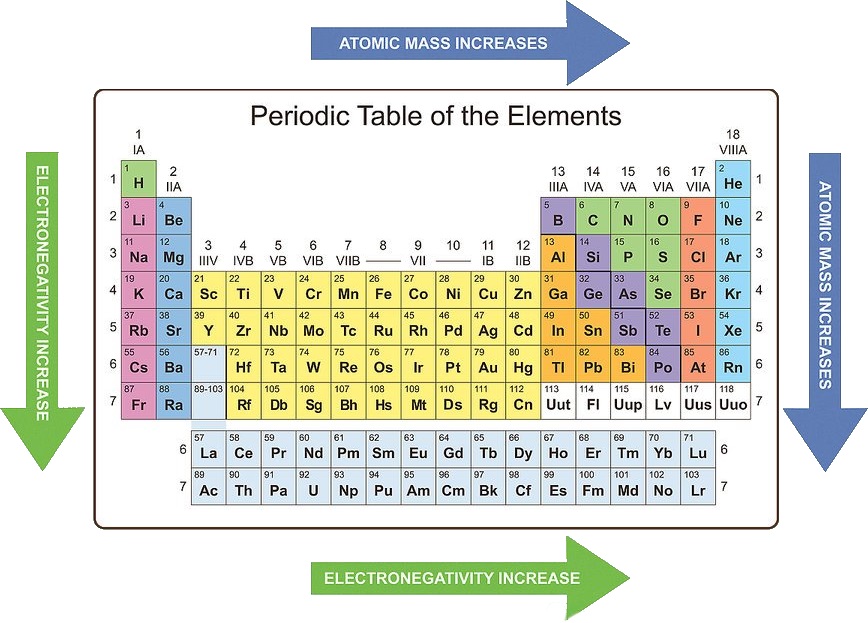
Learn the trends of the periodic table
Knowing periodic trends can help you to recognize the likely chemical properties of an element based on its location in the periodic table. It is important, however, to remember that three groups (noble gases, lanthanides, and actinides) do not follow these trends due to their unique chemistry. Some periodic trends are:
• Atomic mass increases from left to right and from top to bottom.
• Atomic radius decreases from left to right and increases from top to bottom.
• Electronegativity increases from left to right and decreases from top to bottom.
• Ionization energy increases from left to right and decreases from top to bottom.
• Electron affinity increases from left to right and decreases from top to bottom.
• Metallic character decreases from left to right and increases from top to bottom.
Examining Physical Attributes
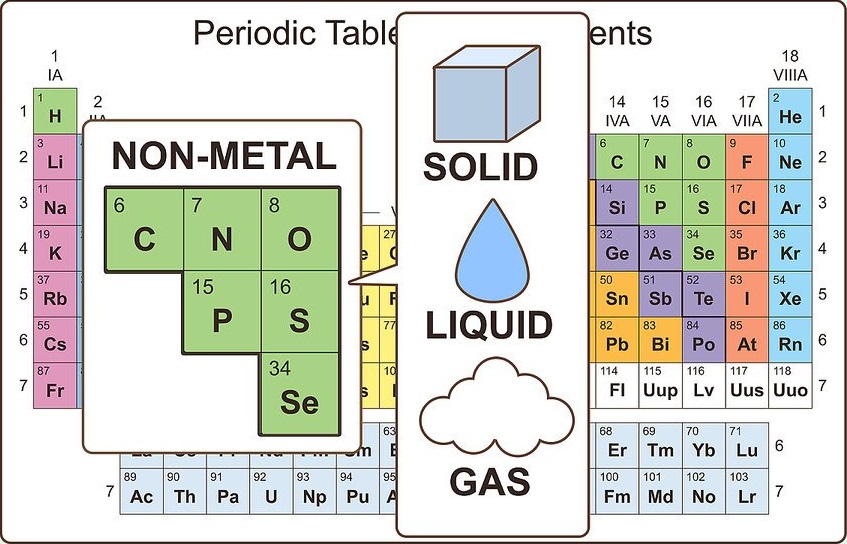
Identify nonmetallic properties.
Nonmetals exist in three physical states at room temperature (solid, liquid, and gas), but are primarily gases at room temperature. Nonmetals are usually dull and brittle when they are solid, and they typically melt and boil at lower temperatures than metals. Nonmetals are also poor conductors of heat and electricity.
The only nonmetal that is a liquid at room temperature is bromine. Carbon has the highest melting point of all elements.
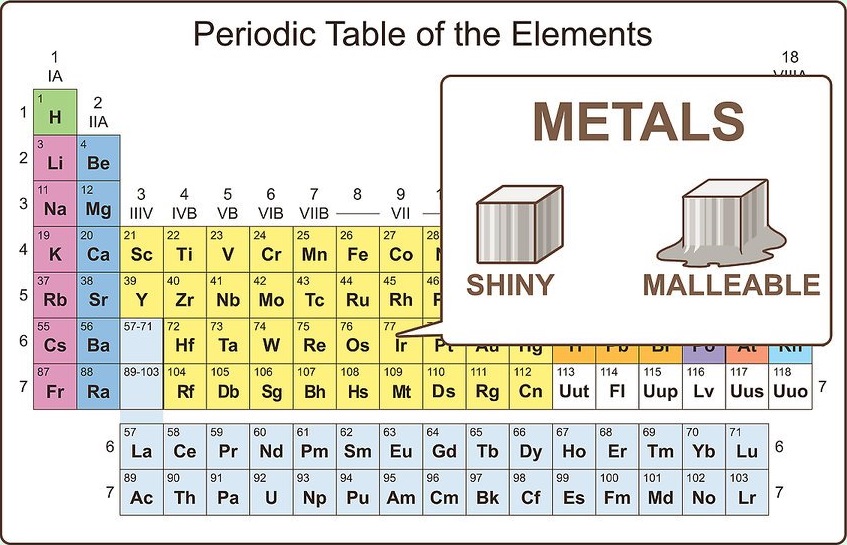
Know the physical properties of metals
Metals are shiny and malleable. They also conduct heat and electricity well. Metals are mostly solid at room temperature, though mercury is a liquid. Metals generally have high melting and boiling points compared to nonmetals.
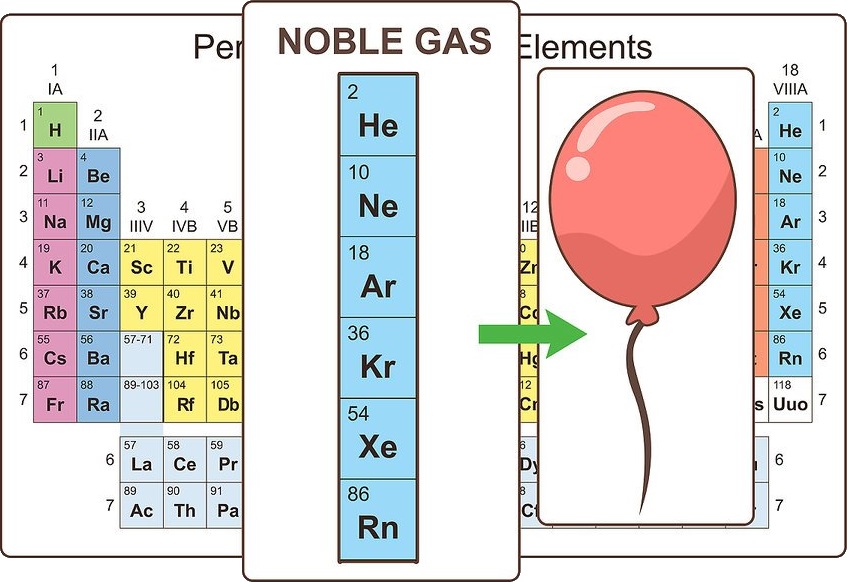
Note noble gases
The elements that make up the far right column are known as the noble gases. They are chemically inert and are all found in the gas phase at room temperature. These gases are used for things like filling balloons and lighting signs.
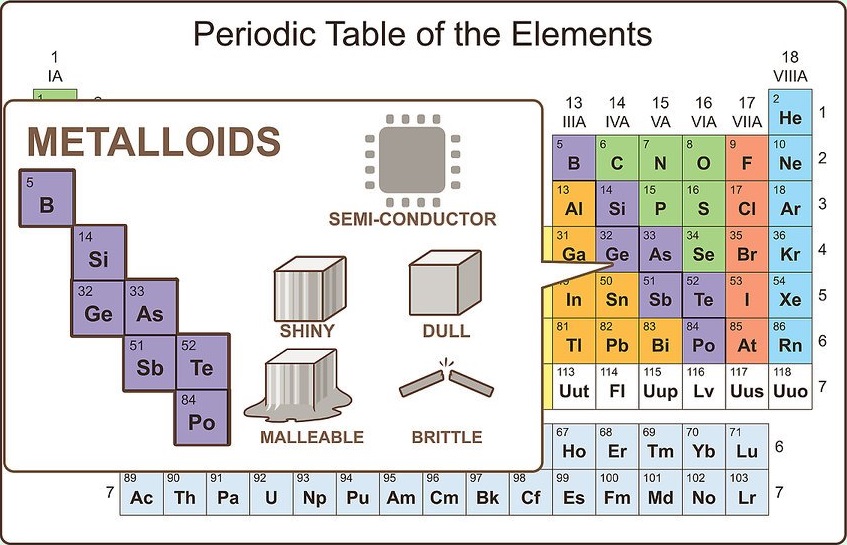
Consider metalloids
Just like metalloids possess chemical properties of both metals and nonmetals, they possess physical properties of both. They are semi-conductors. They can be malleable or brittle. They can also be shiny or dull.
Article source: wikiHow wikiHow is a group effort to create a great resource: the world's largest free how to manual. wikiHow articles help people solve their everyday problems. wikiHow licenses all content under a Creative Commons License. The license allows wikiHow content to be used freely for noncommercial purposes. The Creative Commons License also allows for the creation of derivative works.
Images License: Creative Commons Attribution-NonCommercial-ShareAlike . Uploaded by: Wikivisual - Hi! I'm a bot that adds better images and videos to wikiHow articles.
