Matter is composed of atoms, and atoms are composed of subatomic particles called protons, neutrons, and electrons. Protons and neutrons reside in the atom's nucleus. Protons carry a positive charge (+), neutrons carry no charge. Electrons orbit the nucleus and carry a negative charge (-).
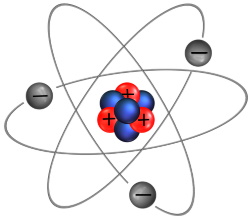
Different elements contain a different number of protons, neutrons, and electrons. Shown above, a lithium atom contains 3 protons, 4 neutrons, and 3 electrons. Normally an atom has the same number of electrons as it has protons, so the charges cancel out so the atom is neutral charge.
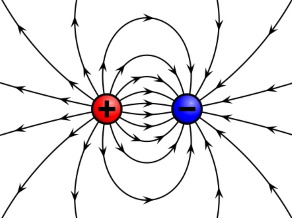
Shown above, particles with opposite charges attract one another. A negatively charged object and a positively charged object attract each other.
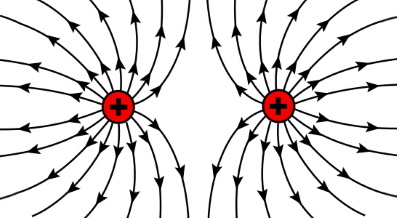
Particles with same charges repel one another. Two positively charged objects or two negatively charged objects repel one another.
A charged object is surrounded by an electrostatic field. It is conventional to represent that field by "lines of force". Arrows on the lines that radiate outward indicate the direction of the force that will be experienced by a same charged object that is brought into that field.
Arrows on the lines of force that point toward the charge indicate the direction of the force that will be experienced by an opposite charged object that is brought into that field.
Lines of force that are closer together indicate the force is greater. At greater distances from a charge the lines get farther apart, indicating that the force is weaker.
The strength of the attraction or repulsion force is determined by Coulomb's law.
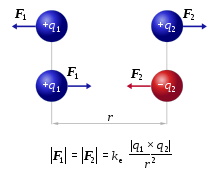
image source en.wikipedia.org/wiki/Coulomb%27s_law
The magnitude of the electrostatic force F between two point charges q1 and q2 is directly proportional to the product of the magnitudes of charges and inversely proportional to the square of the distance between them.
Electric charge is measured in coulombs. A coulomb is the combined electrostatic charge on a certain number of electrons or protons. A coulomb is the amount of charge on 6.2425 x 10^18 electrons.
"Static" means unchanging. Static electricity is a phenomenon caused by a buildup of unbalanced electric charge that is not moving. Contact with another object, or being brought in the proximity of another object may cause charges to move. We are all familiar with the static electric charge caused by dragging our feet across a carpet and then the sudden static discharge caused by grabbing a door knob. This is static electricity phenomenon.
Charging by Friction
Some materials atoms tend to hold on to their electrons more strongly than others. With charging by friction, when two materials are rubbed together, one of them loses electrons and the other gains electrons.
Materials that give up electrons when rubbed become positively charged.
hair
glass
mica
Materials that gain electrons when rubbed become negatively charged.
paper
rubber
plastic
Charging by Contact
One object can become charged by coming into physical contact with another object. Electrons move from one object to the other at the point of contact. The electrons received by an object being charged, depending on how good a conductor of electricity the object is, may distribute themselves evenly over the entire surface of the object. Some materials are good conductors because their electrons, rather than being tightly bound to individual atoms, are free to move from one atom to another.
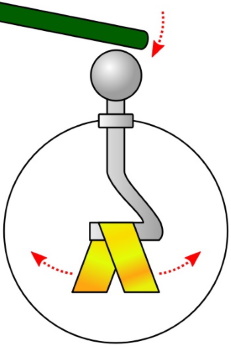
The picture above shows electroscope. An electroscope consists of a metal rod running down the center of an insulating jar and being attached to thin foil leaves. If a negatively charged object touches the top of the rod, the electrons move to the metal rod and down to the foil leaves. Since each leaf has the same charge, they repel each other. This is visible to the experimenter as the leaves move apart.
Charging by Induction
Electrostatic induction is a redistribution of electric charge, not by two objects touching, but by the influence of a charged field in one object.
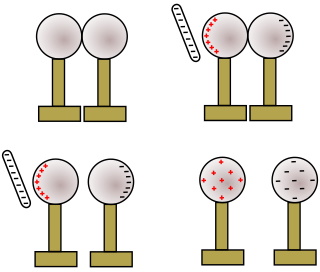
Shown above is an illustration of two metal spheres, both neutrally charged and both insulated from the table.
A negatively charged rod is brought near one sphere. The electrostatic charge field around the negatively charged rod causes free elections in the nearby sphere to be repelled and, because the metal spheres are conductors, they move to the second metal sphere. The first metal sphere acquires a positive charge. The second metal sphere acquires a negative charge.
The two spheres are separated while the charged rod is still near the first sphere.
After the charged rod is removed, since the two metal spheres are separated, charge cannot flow from one sphere to the other to neutralize the charge. The first sphere maintains a positive charge, while the second sphere maintains a negative charge. Because the metal spheres are conductors, the charges distribute themselves evenly over the surface of the spheres.

