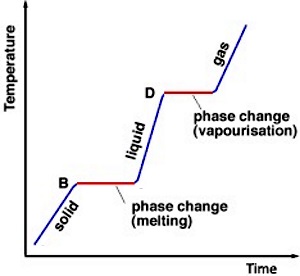
Dutch thermometer pioneer Daniel Gabriel Fahrenheit (1686-1736) had made a curious observation. He noted that the temperature of supercooled water, which immediately changed phase to ice when shaken, shot up to 32 degrees F on his scale. Joseph Black did his own research and he also observed an apparent mismatch between the heating of water and its temperature. When he melted ice, he noticed that although it absorbed heat, its temperature didn't change - in other words it went from ice at 32 degrees F (0 degrees C) to water at 32 degrees F.
It seemed that the heat had somehow combined with the water particles in such a way that it was "hidden" from the thermometer, and so he called it "latent heat". He was even able to measure this latent heat, which is called the "latent heat of fusion" when a substance changes from a solid to a liquid or a liquid to a solid, and the "latent heat of vaporization" when a substance changes from a liquid to a gas.
Black followed up these discoveries by finding that equal masses of different substances need different quantities of heat to change their temperatures by the same amount. This is known as "specific heat". The specific heat of a substance is the energy needed to raise 1g by 1 degree C.
A more general concept is "heat capacity", which is the amount of heat required to change the temperature of a substance. Energy is measured in calories or joules, so specific heat is measured in calories or joules per g degree C. The specific heat of water is 1 calorie/g degree C or 4.186 joules/g degree C.
Setting Temperatures
Fahrenheit's temperature scale was the first to be widely adopted. He set the 0 degree point at the lowest temperature he could achieve, using a mixture of salt and ice. In 1742 Swedish scientist Anders Celsius (1701-1744) proposed that scientific measures of temperature should be made on a fixed scale based on the freezing and boiling of water (at sea level).
He suggested 0 degrees as the temperature at which water boils, and 100 degrees as that at which water freezes, but his pupil inverted the scale and it was adapted across Europe as the Celsius scale. Scientists often prefer to use the Kelvin scale, named for Lord Kelvin (1824-1907). This starts at absolute zero, a theoretical state where no energy at all is present. On the Kelvin scale, which is measured in kelvins (K), water freezes at 273K. 1K = 1C degree = 1.8 degree F.
This is an excerpt from:
Do chemical compounds confuse you? Are you allergic to atoms and muddled by molecules? Then it's time to pick up Incredible Elements a Totally Non-scary to chemistry and why it matters

