Lithium-ion (Li-ion) batteries are one of the most common portable power sources used today. Lithium-ion batteries are rechargeable, very light in weight, and have the highest energy density of any other battery technology available today. Lithium-ion batteries are used in many products such as:
• mobile phones
• computer laptops
• handheld power tools
• appliances like vacuum cleaners
• electric vehicles
• and many other products
Lithium-ion battery (Wikimedia Commons)
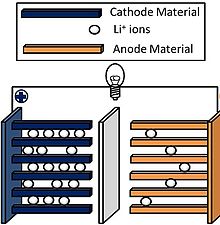
Li-ion batteries are constructed of four components:
• lithium oxide cathode
• graphite anode
• electrolyte composed of salts, solvents and additives
• separator composed of synthetic resin such as polyethylene and polypropylene
In operation, lithium atoms at the cathode give up electrons and become ions. The lithium ions move through the electrolyte towards the anode. A semi-permeable separator keeps the cathode and anode apart, and blocks electron flow, but lets lithium ions pass through. When the lithium ions reach the anode, they attract electrons and become neutrally charged.
The potential difference between the terminals of a cell varies depending upon the materials of the cell, but can be up to 3.6 volts.
Impurities in the materials used to construct a lithium battery can cause it to overheat. Mobile phones and computer laptops made of poorly constructed materials have been known to catch fire.

