In the early 1900's, the plum pudding model was the accepted model of the atom. Proposed in 1904 by J. J. Thomson, the model suggested that the atom was a spherical ball of positive charge, with negatively charged electrons scattered evenly throughout. In that model, the positive charges made up the pudding, while the electrons acted as isolated plums. During its short life, the model could be used to explain why most particles were neutral, although with an unbalanced number of plums, electrically charged atoms could exist.
When Ernest Rutherford began his gold foil experiment in 1909, it is unlikely that anyone would have expected that the plum pudding model would be challenged. However, using a radioactive source, a thin sheet of gold foil, and a phosphorescent screen, Rutherford would uncover something so great that he would later call it "the most incredible event that has ever happened to me in my life" [James, L. K. (1993). Nobel Laureates in Chemistry, 1901-1992. Washington, DC: American Chemical Society.]
The experiment that Rutherford designed is shown below. As you can see in, a radioactive source was placed in a lead container with a hole in one side to produce a beam of positively charged helium particles, called alpha particles. Then, a thin gold foil sheet was placed in the beam. When the high-energy alpha particles passed through the gold foil, they were scattered. The scattering was observed from the bright spots they produced when they struck the phosphor screen.
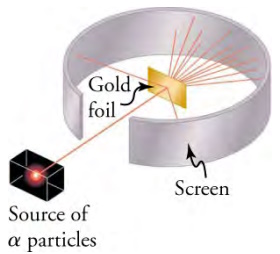
Rutherford's experiment gave direct evidence for the size and mass of the nucleus by scattering alpha particles from a thin gold
foil. The scattering of particles suggests that the gold nuclei are very small and contain nearly all of the gold atom's mass. Particularly
significant in showing the size of the nucleus are alpha particles that scatter to very large angles, much like a soccer ball bouncing off a
goalie's head.
The expectation of the plum pudding model was that the high-energy alpha particles would be scattered only slightly by the presence of the gold sheet. Because the energy of the alpha particles was much higher than those typically associated with atoms, the alpha particles should have passed through the thin foil much like a supersonic bowling ball would crash through a few dozen rows of bowling pins. Any deflection was expected to be minor, and due primarily to the electrostatic Coulomb force between the alpha particles and the foil's interior electric charges.
However, the true result was nothing of the sort. While the majority of alpha particles passed through the foil unobstructed, Rutherford and his collaborators Hans Geiger and Ernest Marsden found that alpha particles occasionally were scattered to large angles, and some even came back in the direction from which they came! The result, called Rutherford scattering, implied that the gold nuclei were actually very small when compared with the size of the gold atom. As shown below, the dense nucleus is surrounded by mostly empty space of the atom, an idea verified by the fact that only 1 in 8,000 particles was scattered backward.
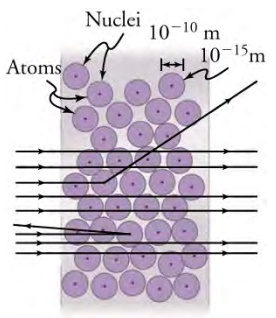
Although the results of the experiment were published by his colleagues in 1909, it took Rutherford two years to convince himself of their meaning. Rutherford later wrote: "It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you. On consideration, I realized that this scattering backwards ... [meant] ... the greatest part of the mass of the atom was concentrated in a tiny nucleus." In 1911, Rutherford published his analysis together with a proposed model of the atom, which was in part based on Geiger's work from the previous year. As a result of the paper, the size of the nucleus was determined to be about 10-15m, or 100,000 times smaller than the atom. That implies a huge density, on the order of 1015 g/cm3, much greater than any macroscopic matter.
Based on the size and mass of the nucleus revealed by his experiment, as well as the mass of electrons, Rutherford proposed the planetary model of the atom. The planetary model of the atom pictures low-mass electrons orbiting a large-mass nucleus. The sizes of the electron orbits are large compared with the size of the nucleus, and most of the atom is a vacuum. The model is analogous to how low-mass planets in our solar system orbit the large-mass Sun. In the atom, the attractive Coulomb force is analogous to gravitation in the planetary system (see below).
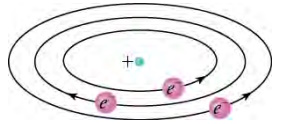
Rutherford's planetary model of the atom incorporates the characteristics of the nucleus, electrons, and the size of the atom. The model was the first to recognize the structure of atoms, in which low-mass electrons orbit a very small, massive nucleus in orbits much larger than the nucleus. The atom is mostly empty and is analogous to our planetary system.
Article source: OpenStax is a nonprofit educational technology initiative based at Rice University. OpenStax's mission is to improve educational access and learning for everyone. Textbooks on OpenStax's site are licensed under a Creative Commons Attribution 4.0 International License.

