Atomic physics deals with the smallest units of elements and compounds. In its study, we have found a relatively small number of atoms with systematic properties, and these properties have explained a tremendous range of phenomena. Nuclear physics is concerned with the nuclei of atoms and their substructures. Here, a smaller number of components-the proton and neutron-make up all nuclei. Exploring the systematic behavior of their interactions has revealed even more about matter, forces, and energy.
Particle physics deals with the substructures of atoms and nuclei and is particularly aimed at finding those truly fundamental particles that have no further substructure. Just as in atomic and nuclear physics, we have found a complex array of particles and properties with systematic characteristics analogous to the periodic table and the chart of nuclides.
The properties of matter are based on substructures called molecules and atoms. Each atom has the substructure of a nucleus surrounded by electrons, and their interactions explain atomic properties. Protons and neutrons - and the interactions between them - explain the stability and abundance of elements and form the substructure of nuclei. Protons and neutrons are not fundamental - they are composed of quarks. Like electrons and a few other particles, quarks may be the fundamental building blocks of all matter, lacking any further substructure.
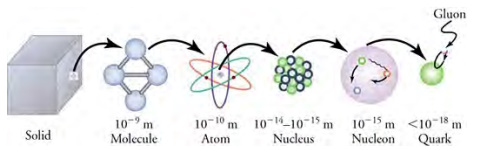
The relative sizes of the components of mater.
Despite the apparent complexity within the universe, there are just four basic forces. These forces are responsible for all interactions known to science: from the very small to the very large to those that we experience in our day-to-day lives. These forces describe the movement of galaxies, the chemical reactions in our laboratories, the structure within atomic nuclei, and the cause of radioactive decay. They describe the true cause behind familiar terms like friction and the normal force. These four basic forces are known as fundamental because they alone are responsible for all observations of forces in nature. The four fundamental forces are gravity, electromagnetism, weak nuclear force, and strong nuclear force.
The gravitational force is most familiar to us because it describes so many of our common observations. It explains why a dropped ball falls to the ground and why our planet orbits the Sun. It gives us the property of weight and determines much about the motion of objects in our daily lives. Because gravitational force acts between all objects of mass and has the ability to act over large distances, the gravitational force can be used to explain much of what we observe and can even describe the motion of objects on astronomical scales. That said, gravity is incredibly weak compared to the other fundamental forces and is the weakest of all of the fundamental forces.
The electromagnetic force is responsible for both electrostatic interactions and the magnetic force seen between bar magnets. When focusing on the electrostatic relationship between two charged particles, the electromagnetic force is known as the coulomb force. The electromagnetic force is an important force in the chemical and biological sciences, as it is responsible for molecular connections like ionic bonding and hydrogen bonding. Additionally, the electromagnetic force is behind the common physics forces of friction and the normal force. Like the gravitational force, the electromagnetic force is an inverse square law. However, the electromagnetic force does not exist between any two objects of mass, only those that are charged.
When considering the structure of an atom, the electromagnetic force is somewhat apparent. After all, the electrons are held in place by an attractive force from the nucleus. But what causes the nucleus to remain intact? After all, if all protons are positive, it makes sense that the coulomb force between the protons would repel the nucleus apart immediately. Scientists theorized that another force must exist within the nucleus to keep it together. They further theorized that this nuclear force must be significantly stronger than gravity, which has been observed and measured for centuries, and also stronger than the electromagnetic force, which would cause the protons to want to accelerate away from each other.
The strong nuclear force is an attractive force that exists between all nucleons. This force, which acts equally between proton- proton connections, proton-neutron connections, and neutron-neutron connections, is the strongest of all forces at short ranges. However, at a distance of 10–13 cm, or the diameter of a single proton, the force dissipates to zero. If the nucleus is large (it has many nucleons), then the distance between each nucleon could be much larger than the diameter of a single proton.
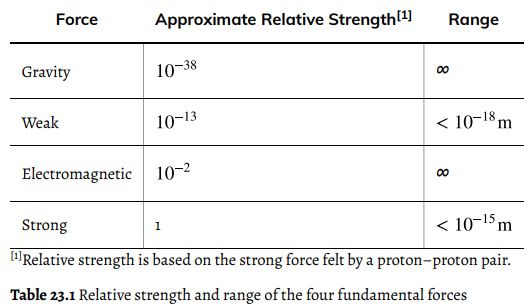
Forces Strength and Range
The weak nuclear force is responsible for beta decay, as seen in the equation Recall that beta decay is when a beta particle is ejected from an atom. In order to accelerate away from the nucleus, the particle must be acted on by a force. Enrico Fermi was the first to envision this type of force. While this force is appropriately labeled, it remains stronger than the gravitational force. However, its range is even smaller than that of the strong force, as can be seen in the table above. The weak nuclear force is more important than it may appear at this time.
Article source: OpenStax is a nonprofit educational technology initiative based at Rice University. OpenStax's mission is to improve educational access and learning for everyone. Textbooks on OpenStax's site are licensed under a Creative Commons Attribution 4.0 International License
.
