Uranium is used as a power source in nuclear reactors and was used to make the first atomic bomb, dropped on Hiroshima in 1945. Uranium is mined as an ore called pitchblende, and consists of several isotopes of different atomic weights and different levels of radioactivity. To be used in fission reactions, the amount of the U-235 isotope must be increased to a level to permit ready fission in a reactor or bomb. This process is called enriching uranium, and there are several ways to do it.

Decide what the uranium will be used for.
Most mined uranium contains only about 0.7 percent U-235, with most of the rest being the comparatively stable isotope U-238. What type of fission reaction the uranium will be used for determines what the level of U-235 must be raised to for the uranium to be used effectively. Uranium used in most nuclear power plants needs to be enriched to a level of 3 to 5 percent 235U-235. (A few nuclear reactors, such as the CANDU reactor in Canada and the Magnox reactor in the United Kingdom, are designed to use unenriched uranium.) Uranium used for atomic bombs and warheads, in contrast, needs to be enriched to 90 percent U-235.
Convert the uranium ore to a gas.
Most of the methods currently in existence for enriching uranium require the ore to be converted to a low-temperature gas. Fluorine gas is normally pumped into an ore conversion plant; the uranium oxide gas reacts with the fluorine to produce uranium hexafluoride (UF6). The gas is then acted on to separate out and gather the U-235 isotope.
Enrich the uranium.
The remaining sections of this article describe the various processes available to enrich uranium. Of these, gaseous diffusion and gas centrifuge are the two most common, but the laser isotope separation process is expected to replace them.
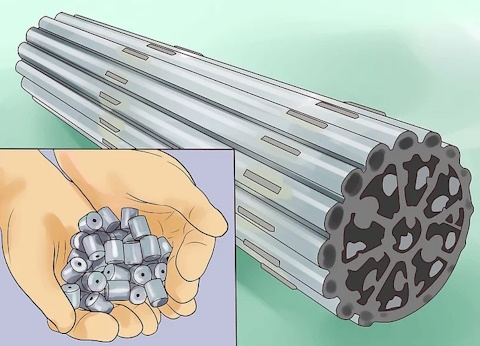
Convert the UF6 gas to uranium dioxide (UO2).
Once enriched, the uranium needs to be converted to a stable solid form for its intended use. Uranium dioxide used as fuel in nuclear reactors is made into centered ceramic pellets encased in metal tubes to make 4m (13.12 foot) long rods.
Force the gas through a porous filter or membrane.
Because the U-235 isotope is lighter than the U-238 isotope, UF6 containing the lighter isotope will diffuse through the membrane faster than the heavier isotope.

Repeat the diffusion process until enough U-235 is collected.
The repeated diffusion is called a cascade. It may take as many as 1,400 passes through porous membranes to get enough U-235 to enrich the uranium sufficiently.
Condense the UF6 gas into liquid form.
Once the gas is sufficiently enriched, it is condensed into a liquid and then stored in containers, where it cools and solidifies for transport to be made into fuel pellets. Because of the number of passes required, this process is energy-intensive and is being phased out. In the United States, only one gaseous diffusion enrichment plant remains, located in Paducah, Kentucky.

Assemble a number of high-speed rotating cylinders.
These cylinders are the centrifuges. The centrifuges are assembled in both series and parallel layouts.
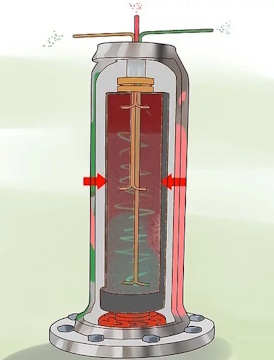
Pipe the UF6 gas into the centrifuges.
The centrifuges use centripetal acceleration to send the heavier 238U-bearing gas to the cylinder
wall and the lighter U-235 bearing gas to the center.
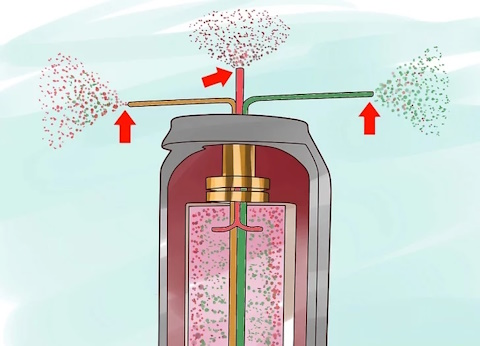
Extract the separated gases.

Reprocess the separated gases in separate centrifuges.
The U-235 rich gases are sent to a centrifuge where still more U-235 is extracted, while the U-235 depleted gas goes to a different centrifuge to extract still more of the remaining U-235. This enables the centrifuge process to extract much more U-235 than the gaseous diffusion process can.
The gas centrifuge process was first developed in the 1940s, but was not brought into significant use until the 1960s, when its lower energy requirements for producing enriched uranium became important. At present, a gas centrifuge processing plant exists in the United States in Eunice, New Mexico. In contrast, Russia currently has four such plants, Japan and China have two each, while the United Kingdom, the Netherlands, and Germany each have one.
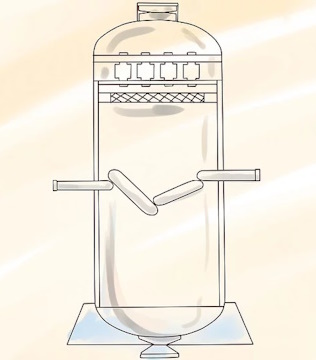
Build a series of stationary narrow cylinders.
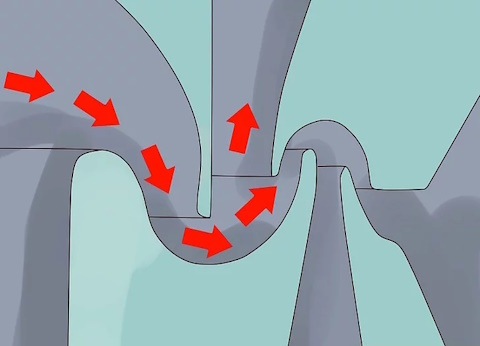
Inject UF6 gas into the cylinders at high speed.
The gas is blown into the cylinders in such a way that it is induced to spin in cyclonic fashion, producing the same kind of separation between U-235 and U-238 as is achieved in a rotating centrifuge.
One method being developed in South Africa injects the gas into the cylinder on a tangent. It is presently being tested with light isotopes such as those in silicon.
Liquefy the UF6 gas under pressure.

Construct a pair of concentric pipes.
The pipes should be fairly tall, with taller pipes enabling more separation of the U-235 and U-238 isotopes.
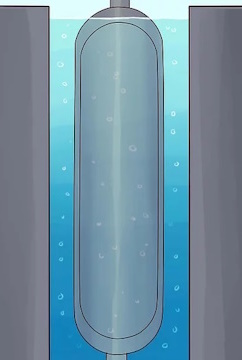
Surround the pipes with a jacket of liquid water. This will cool the outer pipe.
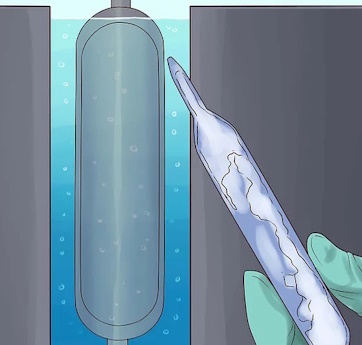
Pump the liquid UF6 between the pipes.
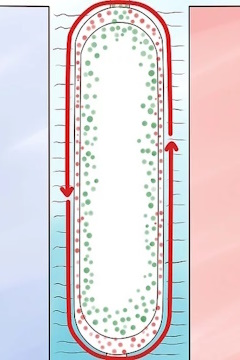
Heat the inner pipe with steam.
The heat will create a convection current in the UF6 that will draw the lighter U-235 isotope toward the hotter inner pipe and push the heavier U-238 isotope toward the colder outer pipe. This process was investigated in 1940 as part of the Manhattan Project, but was abandoned while still in an early stage of development when the more efficient gaseous diffusion process was developed.

Ionize the UF6 gas.
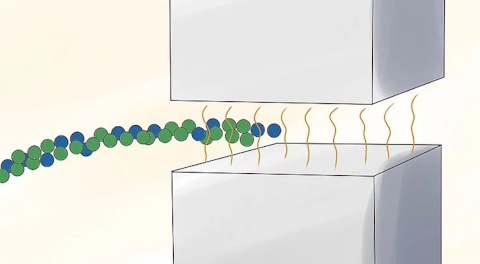
Pass the gas through a strong magnetic field.
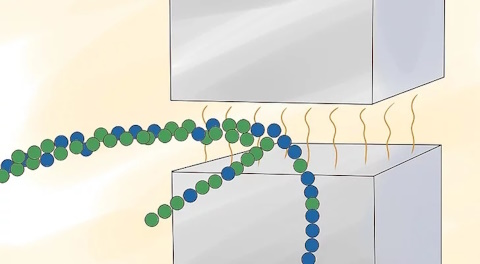
Separate the ionized uranium isotopes by the trails they leave when passing through the magnetic field.
Ions of U-235 leave trails that curve differently than those of U-238. These ions can be isolated to enrich uranium. This method was used to process uranium for the atomic bomb dropped on Hiroshima in 1945 and was also the enrichment method Iraq used in its nuclear weapons program of 1992. It requires 10 times more energy than gaseous diffusion, making it impractical for large-scale enrichment programs.

Tune a laser to a specific color.
The laser light needs to be entirely of a specific wavelength (monochromatic). This wavelength will target only U-235 atoms, while leaving the U-238 atoms untouched.
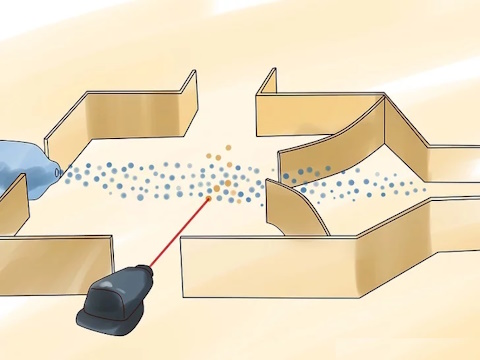
Shine the laser light on the uranium.
Unlike the other uranium enrichment processes, you don’t have to use uranium hexafluoride gas, although most of the laser processes do. You can also use an alloy of uranium and iron as the uranium source, which the Atomic Vapor Laser Isotope Separation (AVLIS) process does.
Extract the uranium atoms with excited electrons. These will be atoms of U-235.
Notes
• In the US it isn't illegal to enrich uranium, but it is illegal to possess enriched uranium.
• Some countries re-process spent nuclear fuel to recover its depleted uranium and plutonium created during the fission process. Reprocessed uranium must be stripped of the U-232 and U-236 isotopes that formed during fission, and if enriched, must be enriched to a higher level than "fresh" uranium because U-236 absorbs neutrons and so inhibits the fission process. For this reason, reprocessed uranium must be kept separate from first time enriched uranium.
• Enriched uranium can ordinarily be reprocessed only once.
• Reprocessed uranium must be kept under heavy shielding, because the U-232 it contains decays into elements that emit a lot of gamma radiation.
• Uranium is actually only weakly radioactive; however, when processed into UF6 gas, it becomes a toxic chemical that reacts with water to form corrosive hydrofluoric acid. (This acid is commonly called "etching acid" because of its use to etch glass.) Uranium enrichment plants thus require the same protective measures as chemical plants that work with fluorine, which include keeping UF6 gas under low pressure most of the time and using extra containment levels in areas requiring higher pressure.
Article source: wikiHow wikiHow is a group effort to create a great resource: the world's largest free how to manual. wikiHow articles help people solve their everyday problems. wikiHow licenses all content under a Creative Commons License. The license allows wikiHow content to be used freely for noncommercial purposes. The Creative Commons License also allows for the creation of derivative works.

