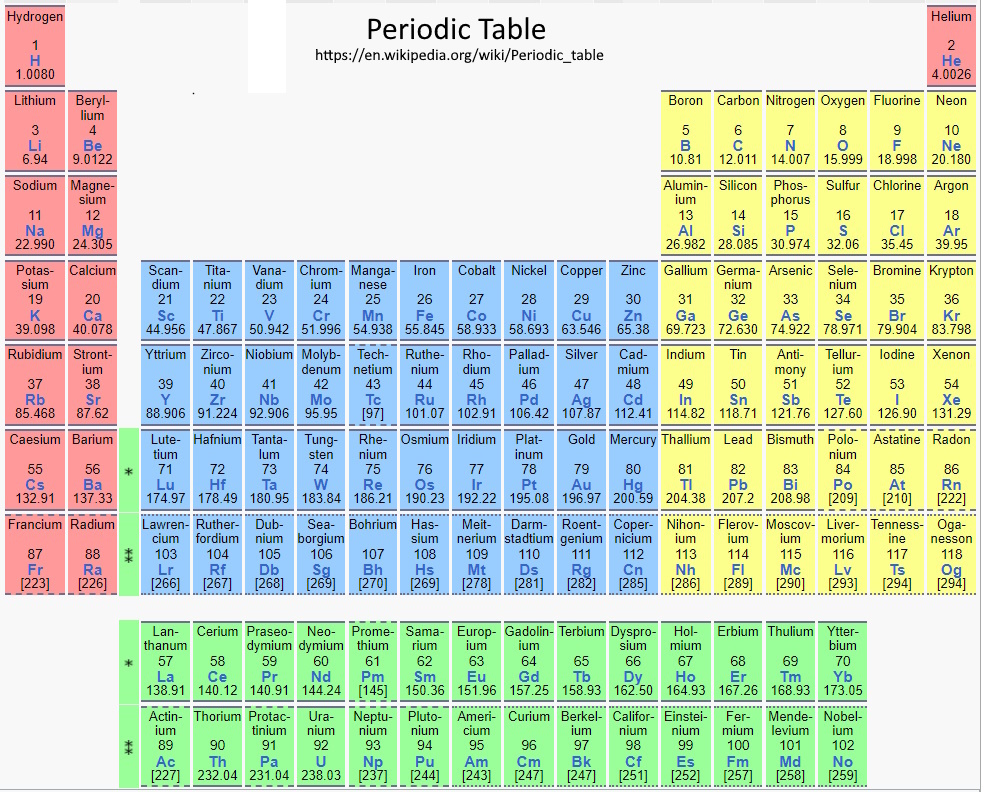
There are currently 118 known elements with 98 are found in nature found in nature and the rest are man-made. The periodic table arranges all the known elements into rows (called periods) and columns (called groups) according to increasing atomic number (the number of protons in the nucleus). Hydrogen with a single proton in it's nucleus is the first element in the table. All elements in the same period have the same number of electron shells. Elements in the same group have the same number of valence electrons.
This arrangement helps chemists understand how an element will interact with other elements and the types of chemical reactions that are likely to be involved in. This arrangement was created by the Russian chemist Dmitri Mendeleev in 1869 when he discovered his periodic law. His original table only contained 63 elements, but the periodic law allowed him to correctly predict the existence and properties of many others. The periodic table is the most important reference in chemistry.
There are a total of 18 groups. The elements in the same group all share similar properties. This reoccurring pattern in their properties is called the "periodic law". Even if an elements is yet to be discovered, its properties can be predicted using the periodic law and the table.
54 elements have been added to the table since Mendeleev first published his Periodic table of 64 elements in 1869. Traditionally a new element added to the table wll be named after a mythological character, a place, a property of the element, or a scientist.

