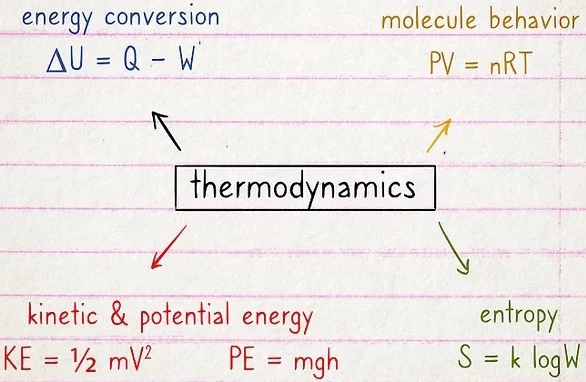
Thermodynamics is all about measuring and understanding how heat and other forms of energy interact with one another. So concepts like energy conversion, molecule behavior, and kinetic/potential energy are key. Entropy is another huge concept. You'll also learn differences between open and closed systems, which changes how heat and energy functions.
Any decent thermodynamics professor will cover the key concepts before they get into the relevant math. Be a diligent note taker in class and ask questions when your teacher is covering the concepts and you'll be fine!
What is the importance of thermodynamics?
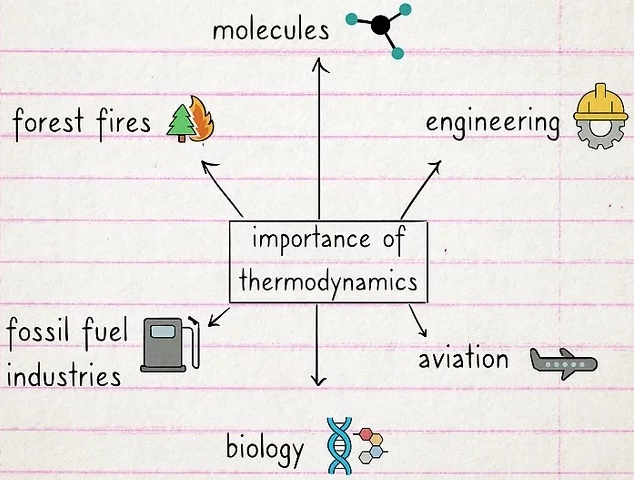
Thermodynamics is essential if you want to understand energy.
If your plans are to build an engine, study the behavior of molecules, or find a more efficient way to prevent forest fires, you need a solid understanding of thermodynamics. Knowledge of thermodynamics is also a prerequisite for jobs in engineering, fossil fuel industries, aviation, and biology.
Even if you don't plan on working in a field related to thermodynamics, you can see the principles everywhere. Thermodynamics are why your appliances work when you plug them into the wall, and why 70 °F (21 °C) water feels cold on your skin, but air of the same temperature is comfortable!
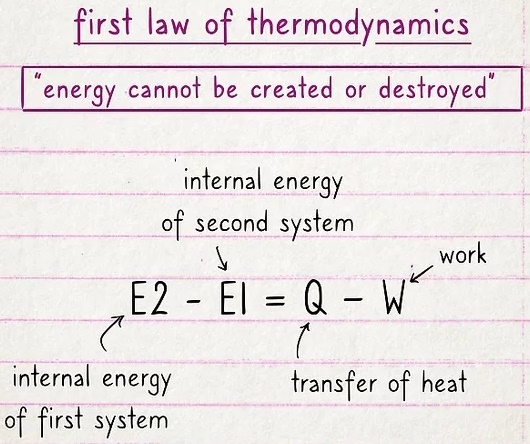
The first law basically says that energy cannot be created or destroyed.
Energy can move or change its form, but it can't appear out of thin air or be completely erased. This law is often expressed by the equation E2 - E1 = Q - W, where the internal energy of one system (E2) minus the internal energy of a second system (E1) is equal to the transfer of heat (Q) minus work (W).
There's actually a law that comes before the first law (the first law was discovered first). It's known as the "zeroth" law. It states that when two objects are in thermodynamic equilibrium with a third object, then the two objects are also in equilibrium with one another.
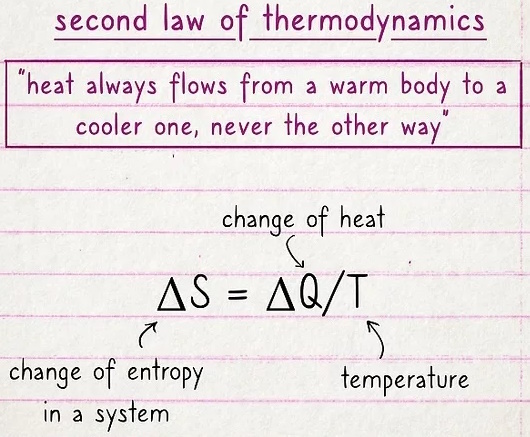
The second law deals with entropy and the movement of heat.
Essentially, if you have a hot object and a cold object next to one another, the heat will transfer from the hot object to the cold one. In fact, the second law states that it can't work the other way. The formula is expressed as ΔS = ΔQ/T, where the change of Q (heat) divided by T (temperature) is equal to the change of entropy (ΔS) in a system.
Entropy is a key concept in thermodynamics. Basically, entropy refers to the amount of energy that's unavailable to do work. A lot of concepts in thermodynamics rely on an understanding of entropy. You'll learn a lot about this early on in class.
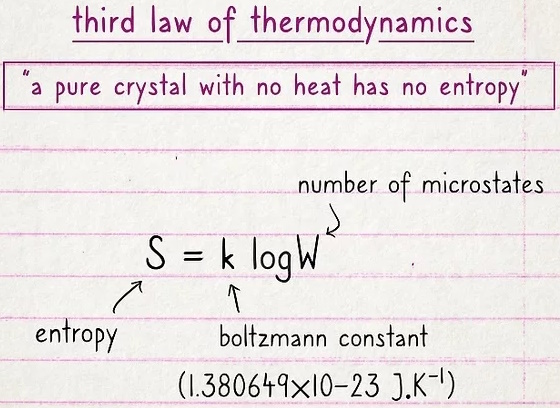
The third law states that a pure crystal with no heat has no entropy.
Entropy is a measure of the disorder of a system. The more disordered a system and higher the entropy, the less of a system's energy is available to do work.
This seems a little random, but it basically boils down to this: if there is no heat, no heat can escape. So, if you have an object with no internal energy and there's no temperature, there's no entropy. There is no formula for the third law of thermodynamics.
Just think of the third law like this: heat has a tendency to leave if a system isn't closed. If there's no heat, there's no transfer. It seems kind of obvious, but it's an essential law when it comes to the behavior of heat and entropy.
Article source: wikiHow wikiHow is a group effort to create a great resource: the world's largest free how to manual. wikiHow articles help people solve their everyday problems. wikiHow licenses all content under a Creative Commons License. The license allows wikiHow content to be used freely for noncommercial purposes. The Creative Commons License also allows for the creation of derivative works.
More Science, Technology, Engineering, and Mathematics Information:
• The Diode
• How to Read a Capacitor's Values
• Zener Diode
• Algebraic Order of Operations
• The Gravitational Force
• Inductors in Series and Parallel
• Vectors and Scalars
• Adding Vectors - Components of Vectors
• Newton's Third Law
• Light Emitting Diode (LED)

