The lead-acid battery has been around since 1859 and is one of the most common form of battery because it's rechargeable and every vehicle has one used to start its engine. Lead acid batteries are heavy because they contain multiple cells, each cell containing two lead plates.
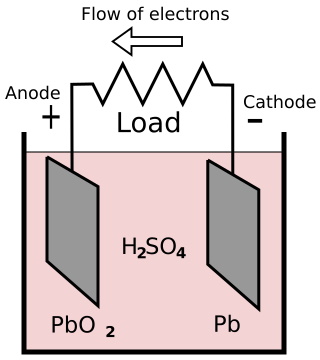
One plate is made of lead (Pb), and the other plate is made of lead-peroxide (PbO2). The plates are immersed in a sulfuric acid (H2 SO4) electrolyte. When sulfuric acid is dissolved in water, it's molecules separate into hydrogen ions (2H+) and sulfate ions (SO4-).
The voltage across the terminals of a cell is 2.2 volts. When a load is connected between the terminals, the sulfate ions travel towards the lead-dioxide cathode (+), and the hydrogen ions travel towards the lead anode (-).
At the cathode, an electron is given up by the sulfate ion causing Pb + SO4 to become PbSO4. At the anode hydrogen ions (2H+) absorb electrons, becoming hydrogen gas. As the cell discharges, the sulfuric acid is consumed and both plates are turned to lead sulfate (PbSO4).
To recharge a lead-acid cell, a reverse voltage is applied across the terminals, positive DC voltage being applied to the anode, this causes the lead sulfate on the plates to react to form sulfuric acid again. During recharging a portion of the lead sulfate remains on the plates (sulfation). Water in the electrolyte is converted into hydrogen and oxygen and released as gasses.
As the cell is repeatedly recharged, sulfation will build-up on the plates and the battery will begin to loose its capacity to restore to a full charge and eventually must be replaced.
More Science, Technology, Engineering, and Mathematics Information:
• Electrical Transformers
• Angle Relationships: Complementary, Supplementary, Adjacent, or Vertical
• Capacitors
• Understand E = mc^2
• What Is an Electronic Circuit?
• Junction Field Effect Transistor (JFET)
• Zener Diode
• Circuit Analysis with Thevenin's theorem
• The Diode
• Mathematics Factoring Basics

